Baeyer–Emmerling indole synthesis
| Baeyer–Emmerling indole synthesis | |
|---|---|
| Named after | Adolf von Baeyer Adolph Emmerling |
| Reaction type | Ring forming reaction |
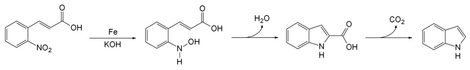
The Baeyer–Emmerling indole synthesis is a method for synthesizing indole from a (substituted) ortho-nitrocinnamic acid and iron powder in strongly basic solution.[1][2] This reaction was discovered by Adolf von Baeyer and Adolph Emmerling in 1869.[3] [4]

Reaction mechanism[edit]
The reaction of iron powder with o-nitrocinnamic acid reduces the nitro group to a nitroso. The nitrogen then condenses with a carbon on the alkene chain with loss of a molecule of water to form a ring. Decarboxylation gives indole.
See also[edit]
References[edit]
- ^ Bayer, A.; Emmerling, A. (1869). "Synthese des indoles" [Synthesis of indoles]. Berichte der deutschen chemischen Gesellschaft. 2 (1): 679–682. doi:10.1002/cber.186900201268.
- ^ Baeyer 5 Archived 2007-08-16 at the Wayback Machine. Pmf.ukim.edu.mk (1997-07-30). Retrieved on 2014-01-10.
- ^ Chamberlain, Joseph Scudder (1921). A Textbook of Organic Chemistry. Blakiston. p. 874.
- ^ Lockyer, Sir Norman (1881). "Indigo and its Artificial Production". Nature. 24 (610): 227–231. Bibcode:1881Natur..24..227H. doi:10.1038/024227c0. S2CID 4100142.
