Atiprimod
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
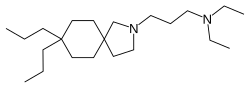
| Formula | C22H44N2 |
| Molar mass | 336.608 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Atiprimod (INN, codenamed SK&F106615) is a substance being studied in the treatment of certain multiple myelomas and other advanced cancers. It may block the growth of tumors and the growth of blood vessels from surrounding tissue to the tumor. This drug is also being researched as a potential treatment for various autoimmune diseases.
It was first developed by GlaxoSmithKline as a potential treatment for rheumatoid arthritis.[1][2][3]
It also had application in the treatment of hyperlipidæmia:[4]
The substance is also known as azaspirane, although this more properly refers to the class of chemicals to which atiprimod belongs.
This compound has also been shown to kill mantle cell lymphoma cells in vitro.[5]
Mechanism of action[edit]
Atiprimod has been shown to inhibit angiogenesis (growth of blood vessels) in a blood vessel model using chicken eggs. It is thought to inhibit the secretion of vascular endothelial growth factor (VEGF), a growth factor that promotes angiogenesis.[citation needed]
Chemistry[edit]
Atiprimod is an amphiphilic compound and a cation at neutral pH.
Synthesis[edit]

The Johnson–Corey–Chaykovsky reaction on 4-Heptanone [123-19-3] (1) gives 2,2-dipropyloxirane [98560-25-9] (2). Treatment with Boron trifluoride etherate [109-63-7] gave 2-Propylpentanal [18295-59-5] (3). Upon acid treatment with Methyl vinyl ketone [78-94-4] (4) this led to 4,4-Dipropylcyclohex-2-enone [60729-41-1] (5). Catalytic hydrogenation of the enone olefin yielded 4,4-Dipropylcyclohexanone [123018-62-2] (7). The Knoevenagel condensation with ethyl 2-cyanoacetate [1187-46-8] (8) led to Cyano-(4,4-dipropyl-cyclohexylidene)-acetic acid ethyl ester [130065-93-9] (8). Conjugate addition of cyanide anion led to ethyl 2-cyano-2-(1-cyano-4,4-dipropylcyclohexyl)acetate, PC45358714 (9). Acid hydrolysis of both the nitrile groups to acids, saponification of the ester, and decarboxylation of the geminal diacid gave 1-(carboxymethyl)-4,4-dipropylcyclohexane-1-carboxylic acid [130065-94-0] (10). Treatment with acetic anhydride gave 8,8-Dipropyl-2-oxaspiro[4.5]decane-1,3-dione [123018-64-4] (11). Condensation with 3-Diethylaminopropylamine [104-78-9] (12) gave the imide and hence, 2-[3-(diethylamino)propyl]-8,8-dipropyl-2-azaspiro[4.5]decane-1,3-dione, PC15634126 (13). Finally reduction of both carbonyl groups with lithium aluminium hydride completed the synthesis of Atiprimod (14).

References[edit]
- ^ Jacobs GS (Spring 2004). "Atiprimod: A New Drug Candidate in Early-Stage Development for Myeloma". Myeloma Today. 5 (10). International Myeloma Foundation. Archived from the original on 2011-07-18. Retrieved 2010-09-30.
- ^ Badger, A. M., Schwartz, D. A., Picker, D. H., Dorman, J. W., Bradley, F. C., Cheeseman, E. N., DiMartino, M. J., Hanna, N., Mirabelli, C. K. (November 1990). "Antiarthritic and supressor cell inducing activity of azaspiranes: structure-function relationships of a novel class of immunomodulatory agents". Journal of Medicinal Chemistry. 33 (11): 2963–2970. doi:10.1021/jm00173a010.
- ^ US4963557 idem Alison M. Badger, WO1989002889 (to Callisto Pharmaceuticals Inc).
- ^ Alison Mary Badger, WO1994025024 (to SmithKline Beecham Corp).
- ^ Wang M, Zhang L, Han X, Yang J, Qian J, Hong S, et al. (June 2007). "Atiprimod inhibits the growth of mantle cell lymphoma in vitro and in vivo and induces apoptosis via activating the mitochondrial pathways". Blood. 109 (12): 5455–5462. doi:10.1182/blood-2006-12-063958. PMID 17317853.
- ^ Badger, A. M., Schwartz, D. A., Picker, D. H., Dorman, J. W., Bradley, F. C., Cheeseman, E. N., DiMartino, M. J., Hanna, N., Mirabelli, C. K. (November 1990). "Antiarthritic and supressor cell inducing activity of azaspiranes: structure-function relationships of a novel class of immunomodulatory agents". Journal of Medicinal Chemistry. 33 (11): 2963–2970. doi:10.1021/jm00173a010.
- ^ US4963557 idem Alison M. Badger, WO1989002889 (to Callisto Pharmaceuticals Inc).
- ^ US 5952365, Dagger RE, Grady CW, "2-[2-(dimethylaminoehtyl]-8,8-diproply-2-azaspiro[4.5]decane dimaleate", issued 4 September 1999, assigned to Anormed Inc.
Further reading[edit]
- Hamasaki M, Hideshima T, Tassone P, et al. (June 2005). "Azaspirane (N-N-diethyl-8,8-dipropyl-2-azaspiro 4.5 decane-2-propanamine) inhibits human multiple myeloma cell growth in the bone marrow milieu in vitro and in vivo". Blood. 105 (11): 4470–6. doi:10.1182/blood-2004-09-3794. PMC 1895034. PMID 15705788.
External links[edit]
- Atiprimod entry in the public domain NCI Dictionary of Cancer Terms
![]() This article incorporates public domain material from Dictionary of Cancer Terms. U.S. National Cancer Institute.
This article incorporates public domain material from Dictionary of Cancer Terms. U.S. National Cancer Institute.
